SGSMedix
| About us | These services include; All aspects of Setting up, Implementing and Improving Quality Systems, including ISO 9001:2000 CE Marking of all Classes of Medical Device or IVD Special Maximum Assistance Minimum Cost Class I Strategy Arranging Notified Body Inspections FDA 510(k), PMA or IDE Submissions Process Validation and Qualification Technical File(s) Preparation, Review or Submission European Authorised Representative Services Review and Advice on Labels, Instructions For Use, Packaging and Advertising Material Design Dossier Compilation, Review and Advice 2nd or 3rd Party Vendor Approvals or Outsource Work Providers Training on any of the above issues. |
|---|---|
| Industry Focus | Bio-technology Products, Medical Equipment |
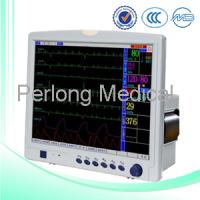
| Products/Services | Medical devices |
| Our Markets | Mid East |
| No. of Employees | Less than 5 People |
| Year Established | 1997 |